By Katherine Chew
 The NIH Public Access Policy (PAC) is part of the Consolidated Appropriations Act of 2008 and was enacted to ensure that the public had access to the published results of NIH-funded research.
The NIH Public Access Policy (PAC) is part of the Consolidated Appropriations Act of 2008 and was enacted to ensure that the public had access to the published results of NIH-funded research.
The NIH PAC requires that all investigators funded by the NIH submit or have submitted for them to the National Library of Medicine’s PubMed Central (PMC) an electronic version of their final, peer-reviewed manuscripts upon acceptance for publication, to be made publicly available no later than 12 months after the official date of publication.
What you need to know
Complying with the NIH PAC is an important skill set for researchers and grant administrators as non-compliance will delay processing of awards if publications arising from NIH grant funding are not in compliance with the policy.
This Research Byte is intended to help researchers and grant administrators understand the basics of the policy and provide tools and resources that can be used to be in compliance with the policy.
Getting started
If your article is funded by an NIH Institute or Center, the policy applies to any manuscript that is:
- Peer reviewed
- Accepted for publication in a journal on or after April 7, 2008;
- and arises from any amount of direct funding from an NIH grant or cooperative agreement; NIH contract or NIH intramural program active or signed in fiscal year 2008 or beyond.
What the Policy does NOT apply to:
- Anything that is NOT peer-reviewed, such as “works for hire,” editorials, short communications, letters to the editor, invited commentary/opinion (unless they go through peer-review)
- Dissertations or books chapters where the publication does not meet the public access definition for a journal.
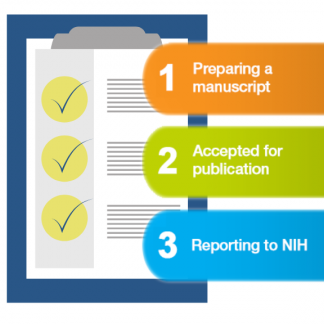
Compliance tasks & responsibilities
In order to comply with the NIH PAC, all NIH-funded investigators who publish their research must carry out the following tasks.
Before publishing – copyright requirements
- Make sure the journal knows the paper is supported by NIH funding, and falls under the NIH public access policy.
- Before an author signs a publication agreement or similar copyright transfer agreement, make sure that the agreement allows the final peer-reviewed manuscript to be submitted to NIH in accordance with the Public Access Policy.
- Be aware of what submission method the journal uses (Methods A, B, C or D)
Upon acceptance – start reporting
- Enter the manuscript information of the paper into your eRA Commons-linked My NCBI My Bibliography account as a “forthcoming” paper.
After publication – submitting paper
- Ensure or confirm that the manuscript is submitted to the NIH Manuscript Submission (NIHMS) system, either by your publisher (Method A, B, D) or yourself (Method C) within the three month (90 day) window of date of publication.
- Reply to all emails from NIH after initial submission if you are the reviewing author to ensure that the manuscript is processed within PMC in a timely manner.
After publication – documenting compliance
- An eRA Commons linked My NCBI (My Bibliography) is required to report papers, when electronically submitting progress reports using the Research Performance Progress Report (RPPR).
- When citing a paper in NIH applications, proposals, and progress reports, include the PMCID (PubMed Central ID) at the end of the full citation
About the author

Katherine Chew
Katherine Chew, MLS, is an Associate Librarian and the Research/Outreach Services Librarian for the Health Sciences Libraries at the University of Minnesota.
She provides research support and consultation to University of Minnesota researchers in the Academic Health Center including support for NIH Public Access Policy Compliance and other federal funder public access requirements.
Katherine shares these helpful resources on the NIH Public Access Policy:
She welcomes you to contact her at chewx002@umn.edu for help with NIH Public Access Policy compliance.